![Analysis of Hückel's [4n + 2] Rule through Electronic Delocalization Measures | The Journal of Physical Chemistry A Analysis of Hückel's [4n + 2] Rule through Electronic Delocalization Measures | The Journal of Physical Chemistry A](https://pubs.acs.org/cms/10.1021/jp803745f/asset/images/medium/jp-2008-03745f_0002.gif)
Analysis of Hückel's [4n + 2] Rule through Electronic Delocalization Measures | The Journal of Physical Chemistry A
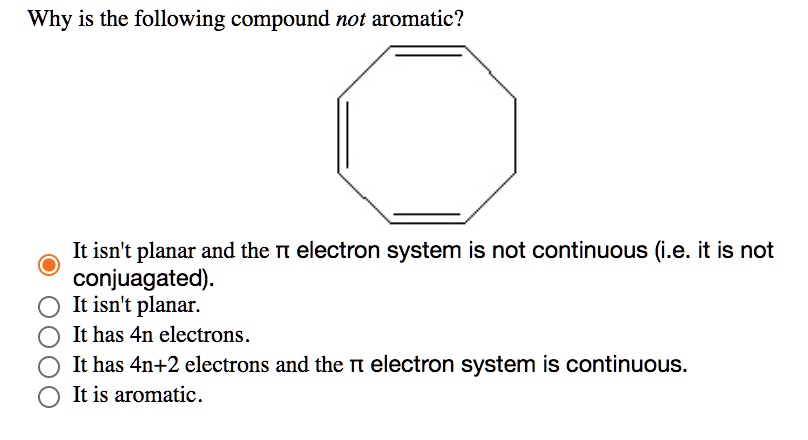
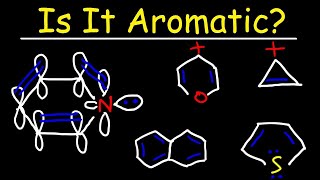
SOLVED: Why is the following compound not aromatic? It isntt planar and the TT electron system is not continuous (i.e: it is not conjuagated): It isn't planar: It has 4n electrons It

Classics Illustrated: Clar's Sextet and Hückel's (4n + 2) π-Electron Rules | The Journal of Physical Chemistry C
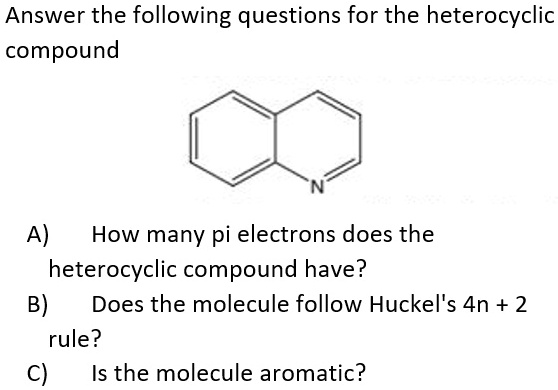

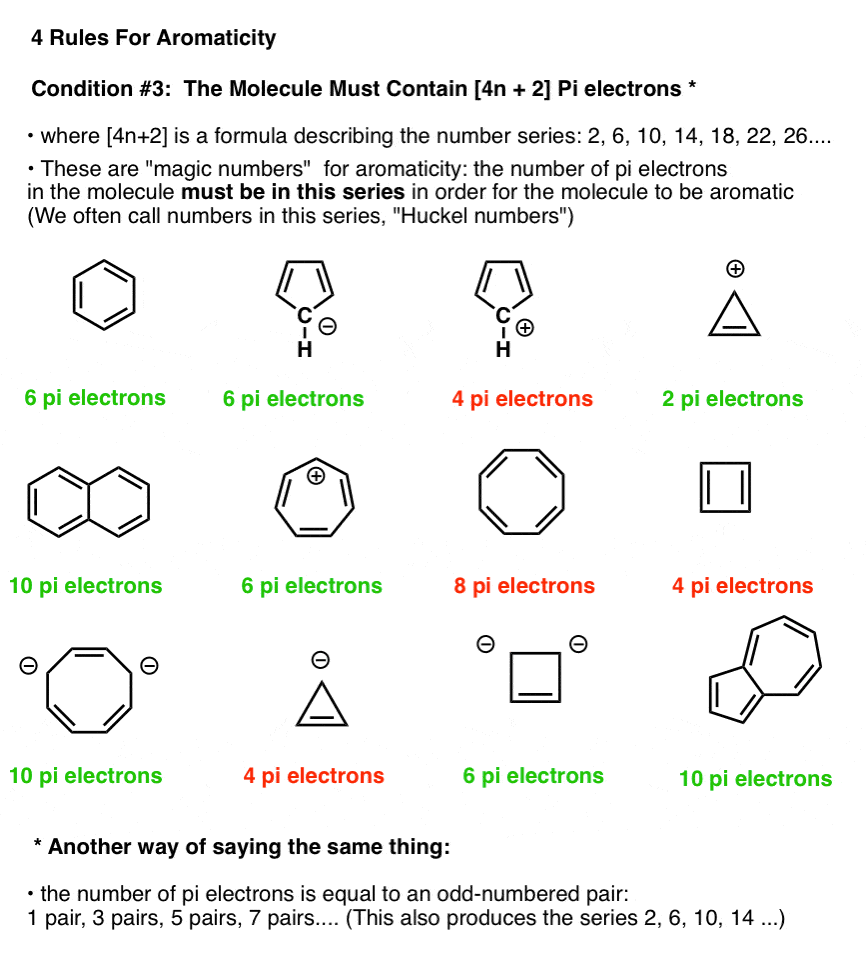

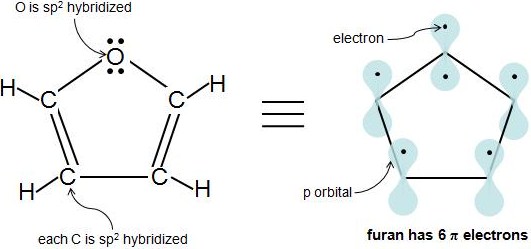.jpg?revision=1&size=bestfit&width=369&height=173)


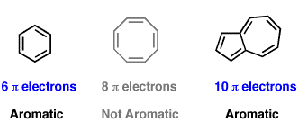

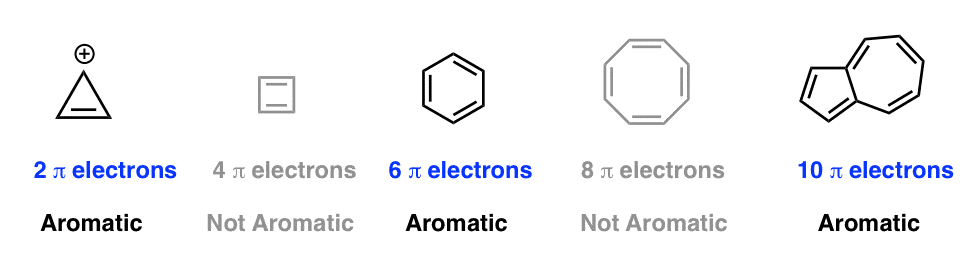

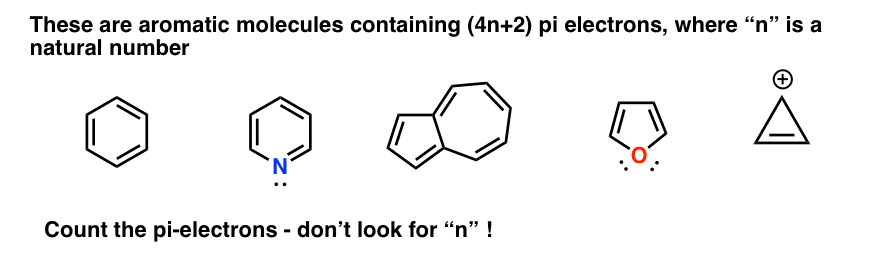
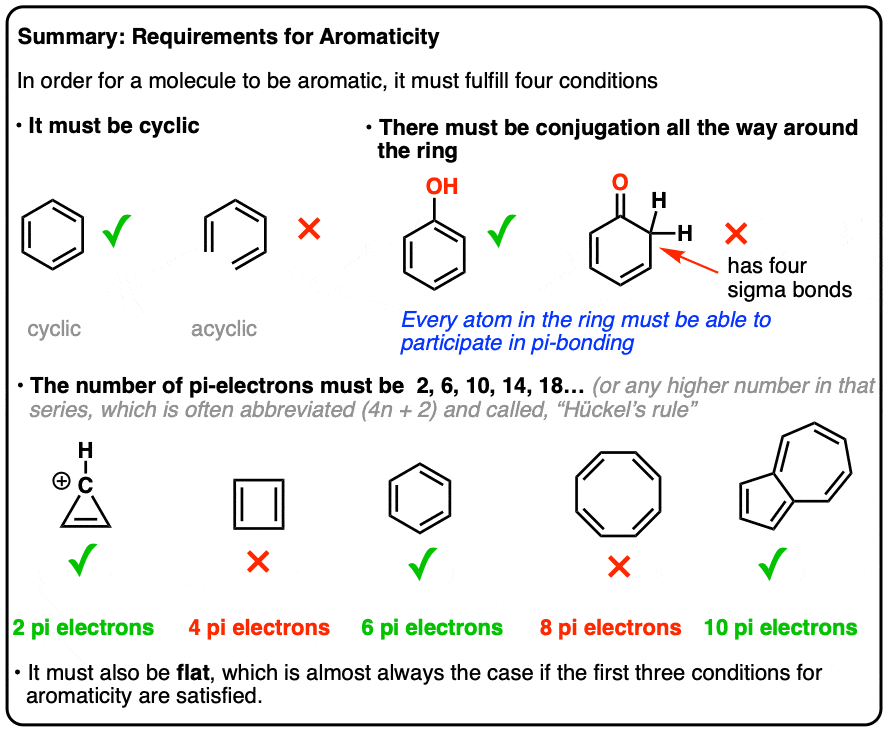

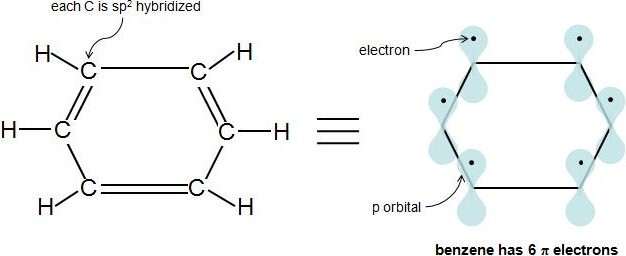.jpg?revision=1)
