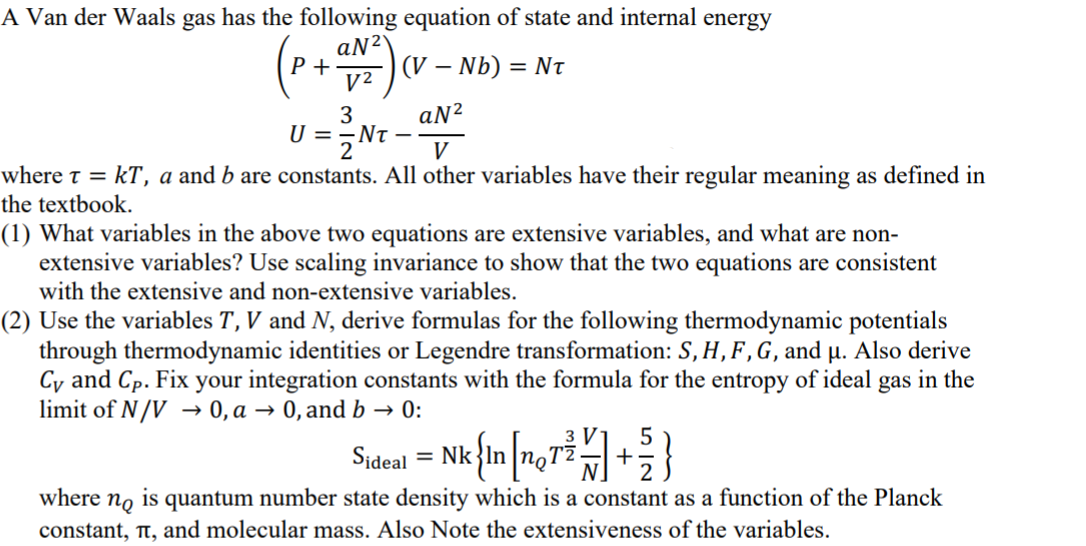
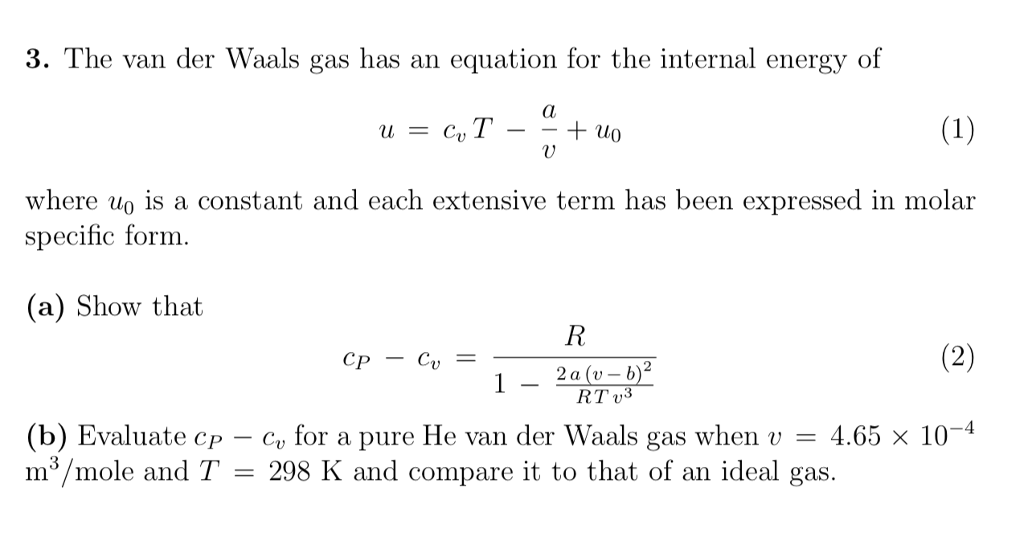
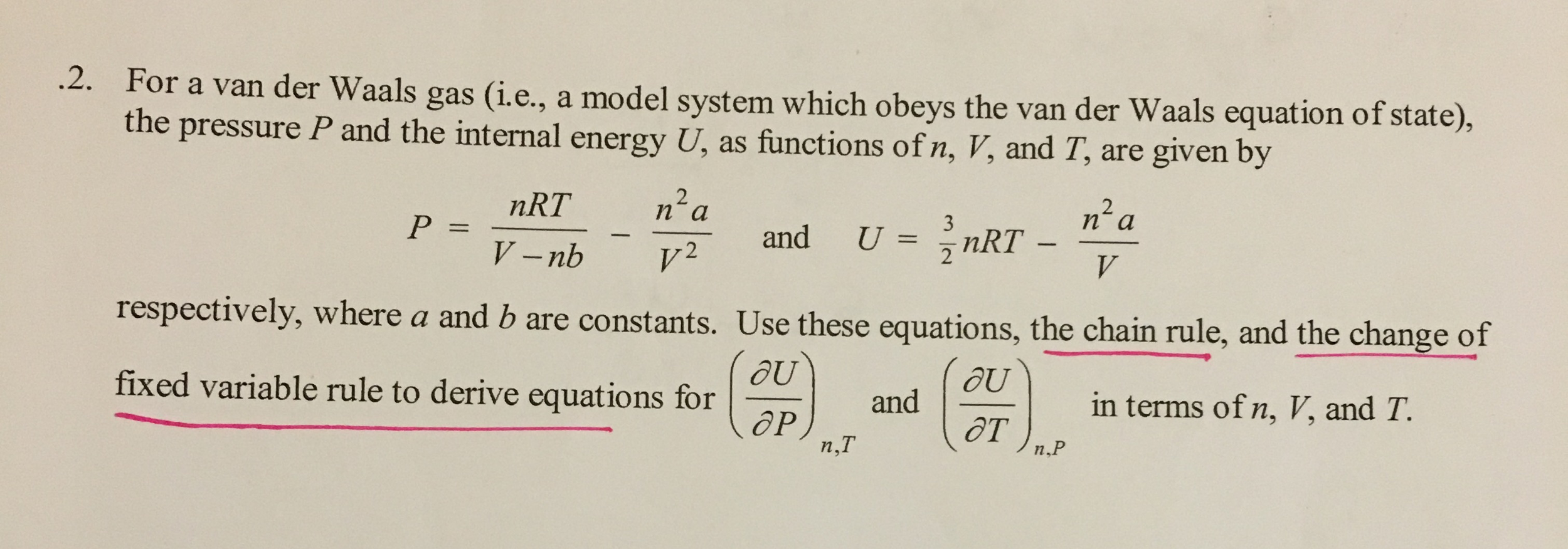
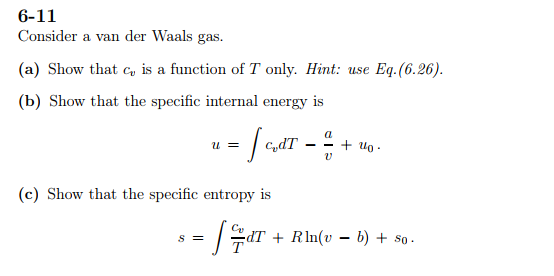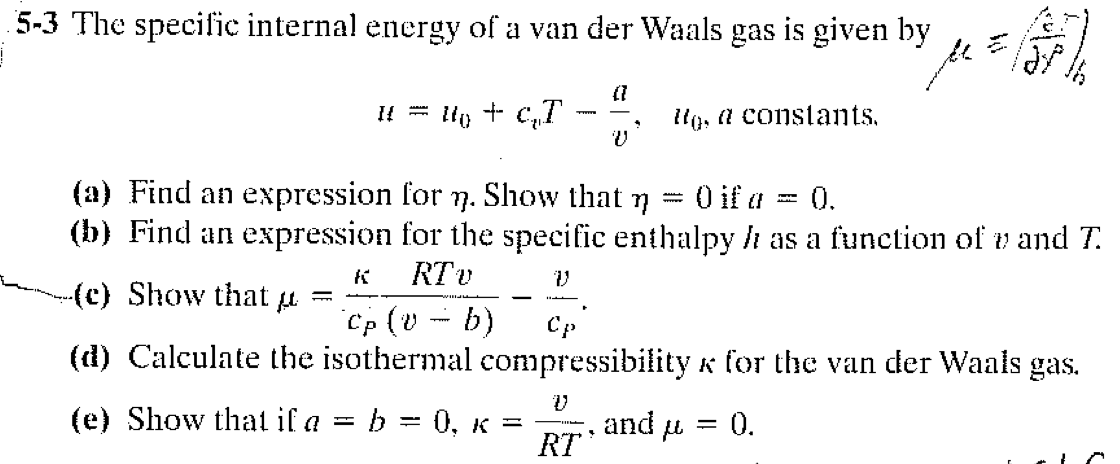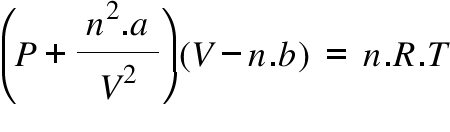Determine the following when a gas obeys Van der Waals' equation, - Sarthaks eConnect | Largest Online Education Community
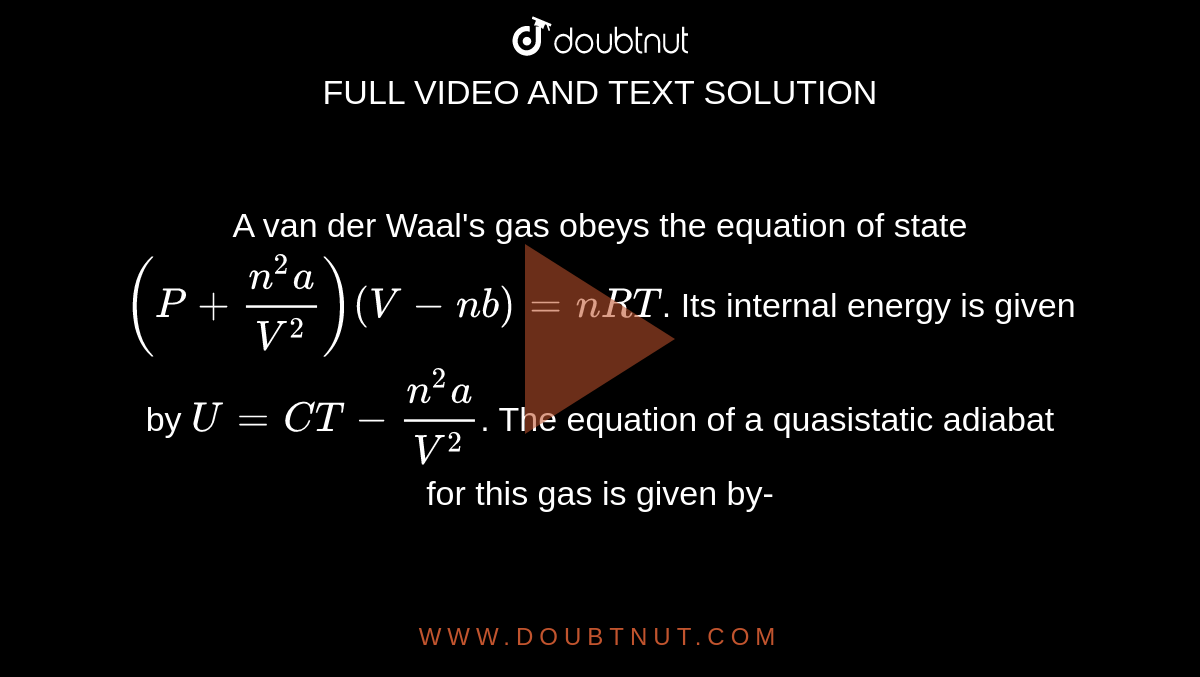
A van der Waal's gas obeys the equation of state (P+(n^(2)a)/(V^(2)))(V-nb)=nRT. Its internal energy is given by U=CT-(n^(2)a)/(V^(2)). The equation of a quasistatic adiabat for this gas is given by-

SOLVED: The partition function for an interacting gas is V Nb mkg 3N/2 Z = exp ( N? a? [VkgT ) N 2th? where a and b are constants Show that the
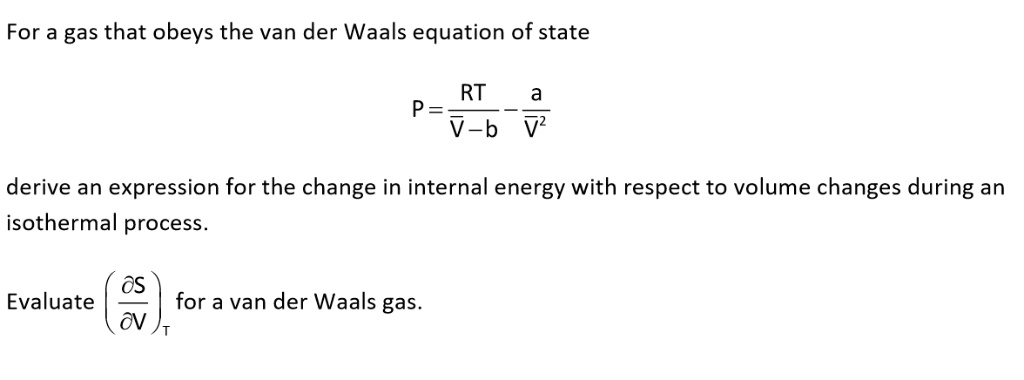
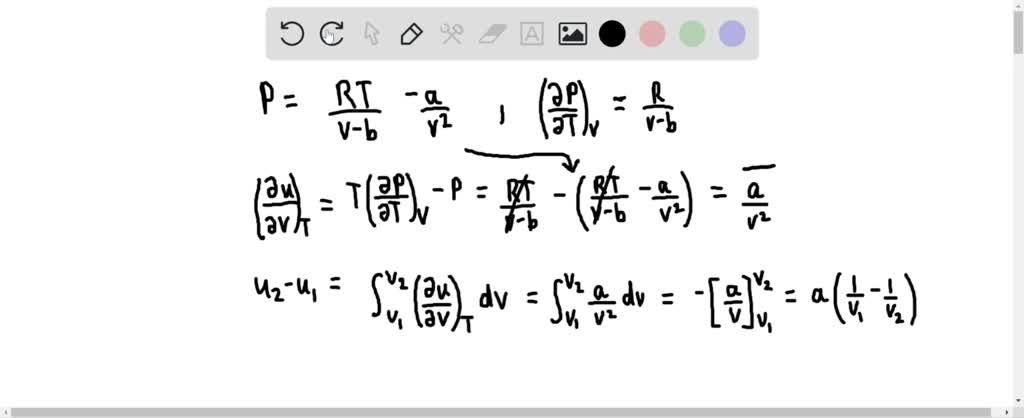
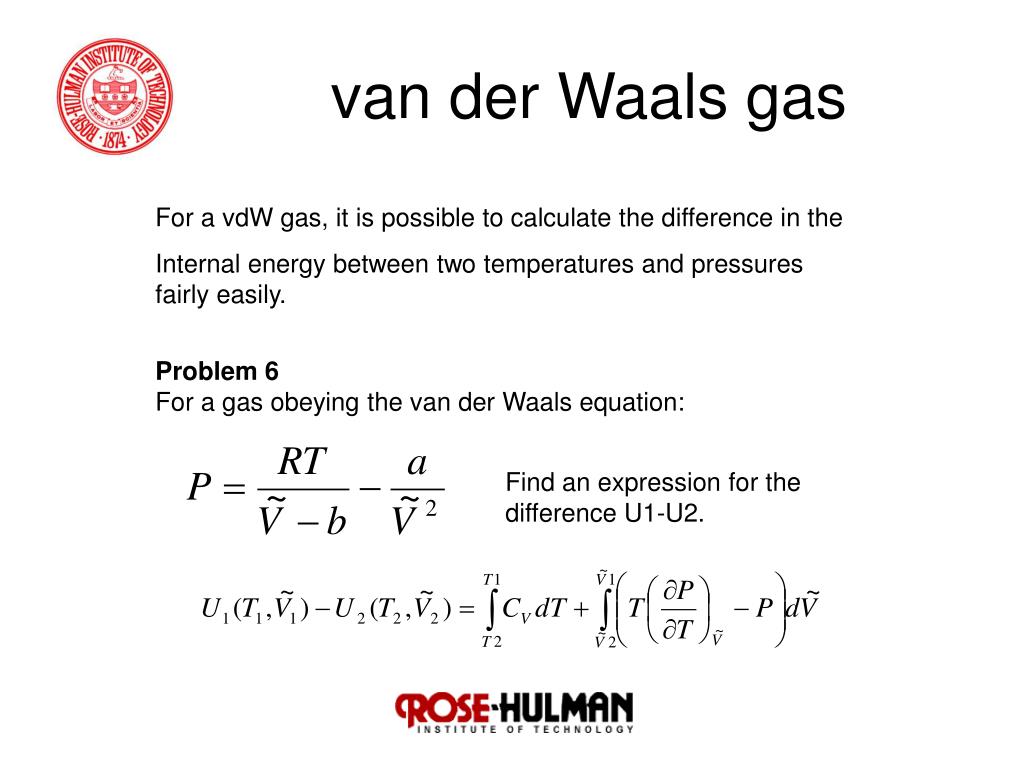
SOLVED: For a gas that obeys the van der Waals equation of state RT P= V-b derive an expression for the change in internal energy with respect to volume changes during an

A van der Waal's gas obeys the equation of state (P+(n^(2)a)/(V^(2)))(V-nb)=nRT. Its internal energy is given by U=CT-(n^(2)a)/(V^(2)). The equation of a quasistatic adiabat for this gas is given by-
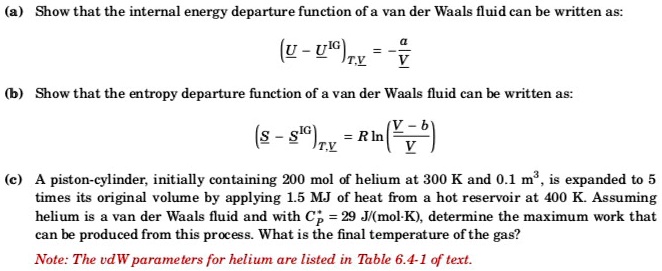
SOLVED: Show that the internal energy departure function of van der Waals fluid can be written as: (u-pc)zx V (b) Show that the entropy departure function of van der Waals fluid can

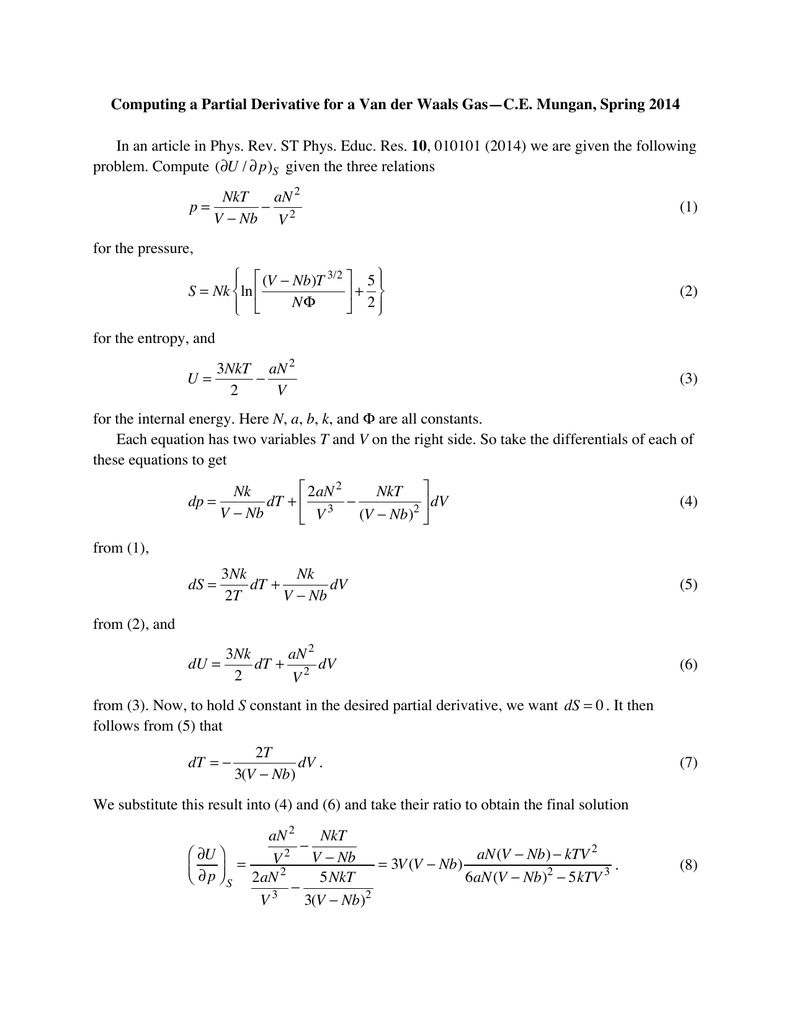
How come delta U is not equal to 0 in an isotherm expansion with a van der Waals gas but it is when an isotherm expansion (or compression, either way) is performed

A van der Waal's gas obeys the equation of state (P+(n^(2)a)/(V^(2)))(V-nb)=nRT. Its internal energy is given by U=CT-(n^(2)a)/(V^(2)). The equation of a quasistatic adiabat for this gas is given by-