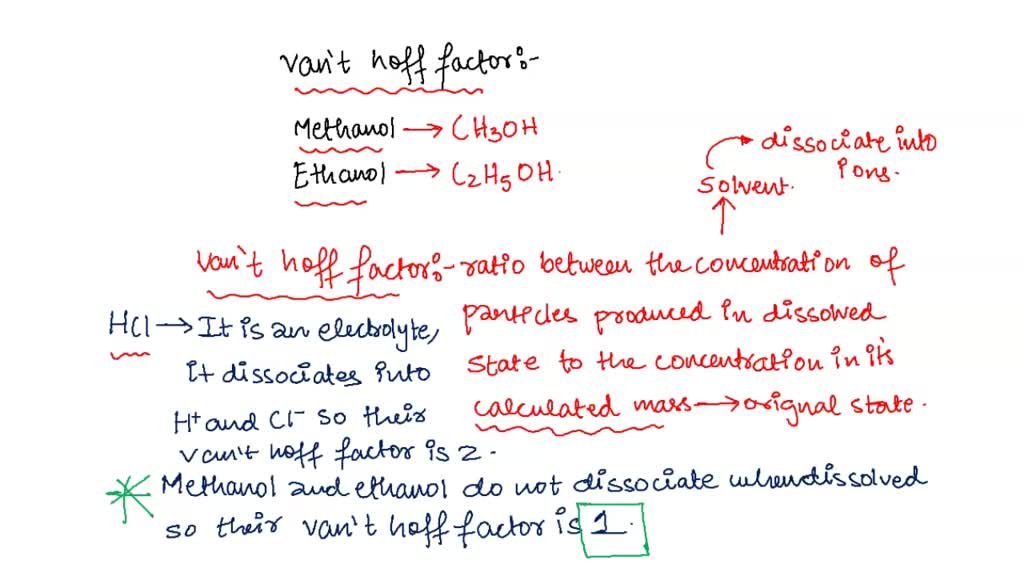
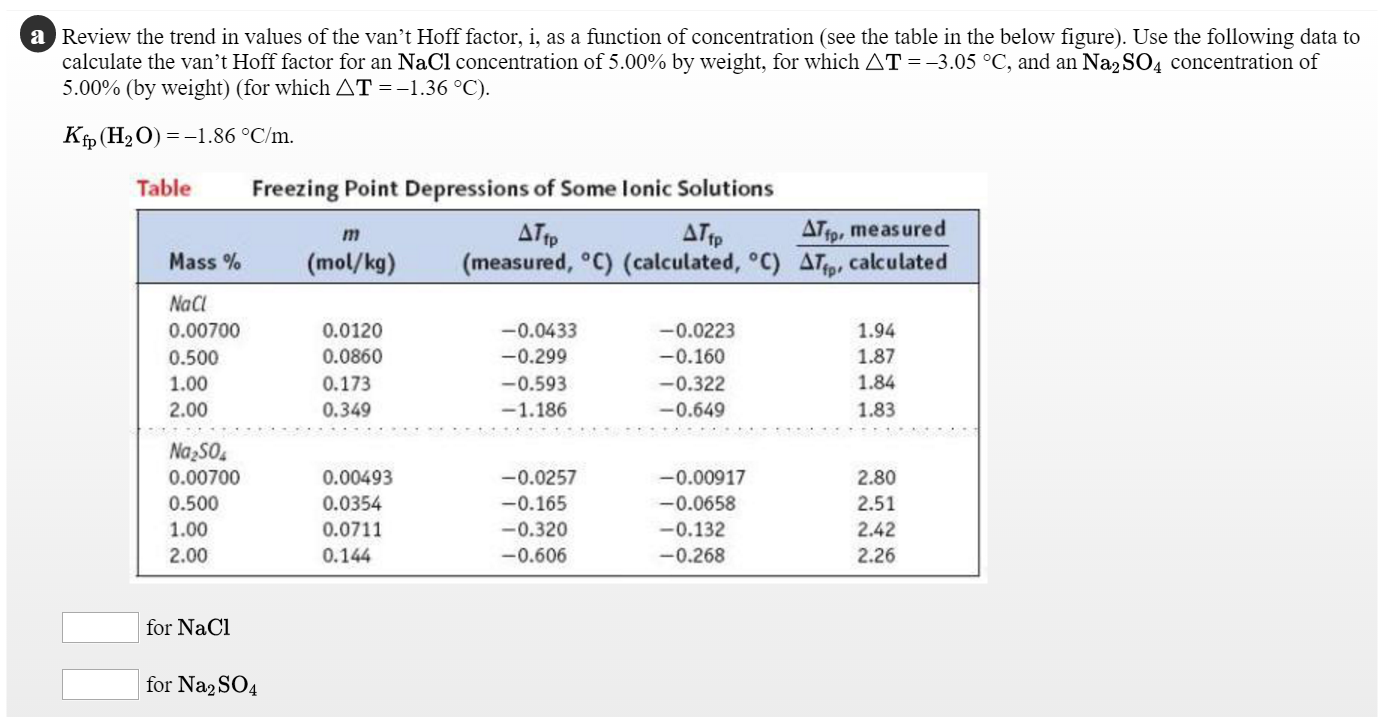
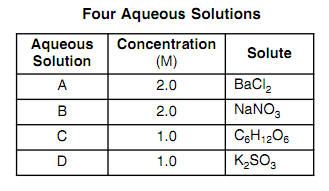
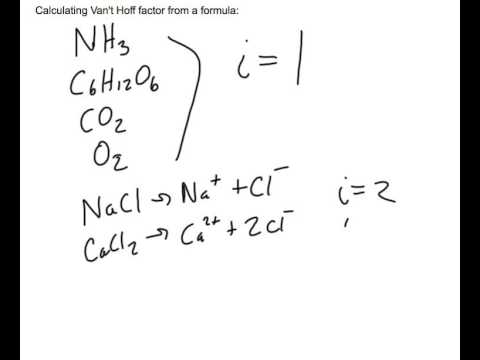
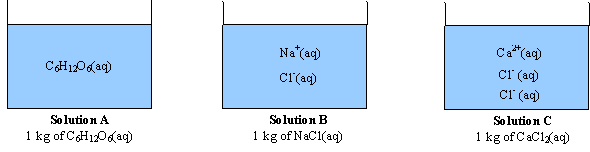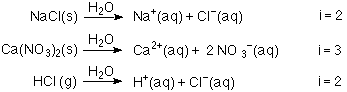128. Which one has same Van't Hoff factor i as that of Hg2Cl2: (1) NaCl (3) Al(NO3)3 (2) Na2SO4(4) Al,(SO4)3

At what temperature would 1.3 m NaCl freeze, given that the van 't Hoff factor for NaCl is 1.9? Kf for water is 1.86 degrees C/m. | Homework.Study.com

Welcome to Chem Zipper.com......: The van't Hoff's factor for 0.1 M Ba(NO3)2 solution is 2.74. the degree of dissociation is.

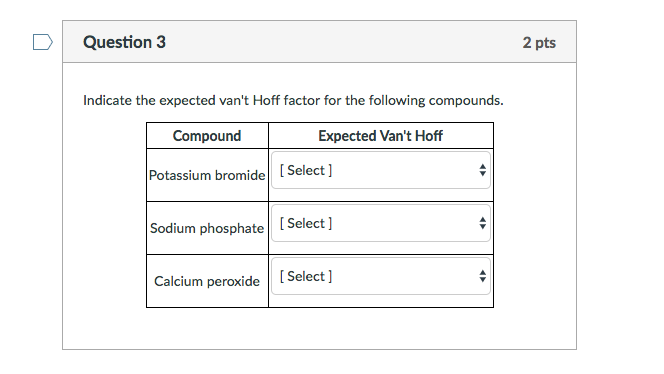
SOLVED: QUESTION 13 What is the Vant Hoff factor of the following compound or molecule in solution Na2CO3 KOH NH4 CI D.3 C6H12 06